Process Chromatography in Wastewater Treatment
Treatment of Wastewaters Containing Fertilizer Nutrients / Metal Ions
Problem
Today, municipalities use process chromatography in wastewater treatment at centralized plants. This water often carries nitrogen and phosphorus from human waste, food, soaps, and detergents. After treatment, plants discharge the water into local rivers. However, even treated water can still release high levels of nitrogen and phosphorus, adding to water pollution.
According to the U.S. Environmental Protection Agency, removal rates differ widely. Some facilities rely on advanced systems that cut nitrogen and phosphorus more effectively than conventional methods. Still, upgrading treatment plants costs municipalities and ratepayers a great deal. On the other hand, these upgrades often pay for themselves over time because they boost efficiency and reduce environmental impact. As a result, communities across the country continue to explore new strategies for lowering nitrogen and phosphorus in wastewater.
Other Sources of Contamination
Wastewater plants are not the only problem. Large amounts of nitrogen, phosphorus, and metals also flow into waterways from stormwater runoff, broken septic systems, ballast water, mining sites, and hydraulic fracturing flowback. These cases require on-site cleanup because they contain phosphates, humic acids, and metals such as arsenic, zinc, silica, copper, lead, and selenium.
Sorbtech’s Solution
Sorbtech meets these challenges with granular activated aluminas used in process chromatography. These materials work as selective adsorbents that remove or recover metal ions. Their performance depends on factors such as pH, ionic strength, and metal ion speciation. Activated alumina can be used in both point-of-entry and point-of-use systems, and its effectiveness depends on contaminant type, alumina characteristics, device design, and water quality. Flow rate and contaminant load also affect overall capacity.
General Adsorption Rules
-
Anion adsorption works best between pH 4–8, with an optimum near 5.5.
-
Cation adsorption occurs across a broader range, from pH 5–12.
-
Oxy-anions in solution often improve cation adsorption.
-
Chelates and complex formation strongly influence both selectivity and capacity.
Predicting Performance
Researchers combine alumina surface chemistry data with aqueous chemical equilibrium models to predict removal efficiency. These tools help design systems, improve accuracy, and ensure better results in real-world water treatment.
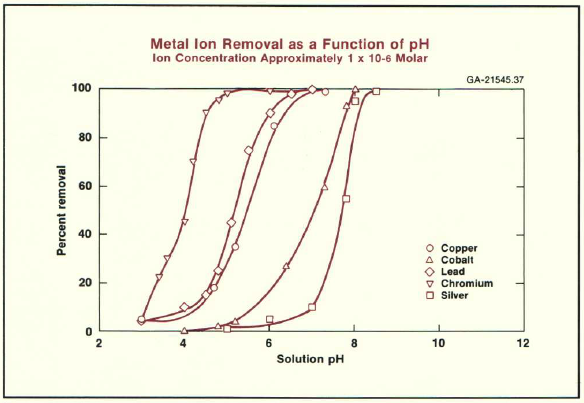
The figure above presents data from Alcoa Labs in New Kensington, PA, illustrating how activated alumina removes metal ions at different pH levels. Researchers gathered this information under dilute solution conditions, using 1 gram per liter of solids and 1 micromolar metal ion concentrations.
General Guidelines for Metal Ion Adsorption with Activated Alumina
-
Anion adsorption works best between pH 4 and 8, with the optimum near pH 5.5.
-
Cation adsorption occurs across a wider pH range, with an effective window from about 5 to 12.
-
Oxy-anions in solution can enhance cation adsorption, making removal more efficient.
-
Complex or chelate formation strongly influences both selectivity and adsorption capacity for anions and cations.
Predicting Removal Efficiency
To better anticipate real-world performance, scientists combine alumina surface chemistry data with aqueous chemical equilibrium models. These models allow for more accurate predictions of how activated alumina will behave under different conditions, guiding system design and improving overall treatment effectiveness.
Alumina in Water Treatment – surface adsorption selectivity series
Cations
Th (II), Al(III, U(IV) > Zr(II), Ce(IV) > Fe(III), Ce(III) > Ti(III) > Hg(II) > UO2(II) > Pb(II) > Cu(II) > Ag(I) > Zn(II) . Co(II), Fe(II) > Ni(II) > Ti(I) > Mn(II)
Anions
OH- > PO43- > C2O42- > F- > SO32-, Fe(CN)64-, CrO42- > S2O32- > Fe(CN)63-, Cr2O72- > NO2-, CNS- > I- > Br- > Cl- > NO3- > MnO4- > ClO4-, CH3COO- > S2-
Key Features of Activated Alumina Systems
-
Durable media: The adsorbent remains stable and does not break down during backwashing.
-
Versatile performance: Operates effectively across a wide range of water chemistries.
-
Safe transport: Shipped in non-hazardous containers designed for land, air, and sea transportation.
-
Responsible disposal: With proper post-treatment, spent media can be safely landfilled as non-hazardous waste.
-
Compact design: The small footprint makes integration easy in existing water treatment plants, including refineries and industrial facilities.
-
Cost efficiency: Low operational costs with no moving parts; systems are available in many size options to match different process needs.
-
Customizable chemistry: Surface properties—such as polarity, acidity or basicity, porosity, and surface area—can be tailored for specific applications.

Image from EPA’s Office of Research and Development and Office of Water
Dual adsorbent bed systems are most desirable, whether in series or parallel. A highly adsorptive activated alumina media and chemical treatment system, combine to provide a simple, safe, and compact process. The addition of automatic controls provides minimum attendance by operations personnel.
Schedule a phone or video conference with your Account Manager to discuss in more detail.
The information contained in this data sheet is believed to be true and accurate but is presented for guidance only. Risks and liability for use of the products or application of the suggestions described are assumed by the user. Any recommendations or suggestions are made without warranty or guarantee.