Clarion™ Mini Spin Columns
For protein desalting and buffer exchange of samples to 100uL
Our mini spin columns feature rapid desalting, buffer exchange, and removal of small molecular weight impurities using only a microcentrifuge.
Proteins, oligos, or nano-particles are simultaneously purified, desalted, and eluted into pure water in a single action.
Clarion Mini Spin Columns Feature:
- Removal of up to 99.999% salts, dyes, haptens, and other small molecules
- Process samples up to 100 µL in under 5 minutes
- Sterile packed, pre-swollen with purified water and ready-to-use
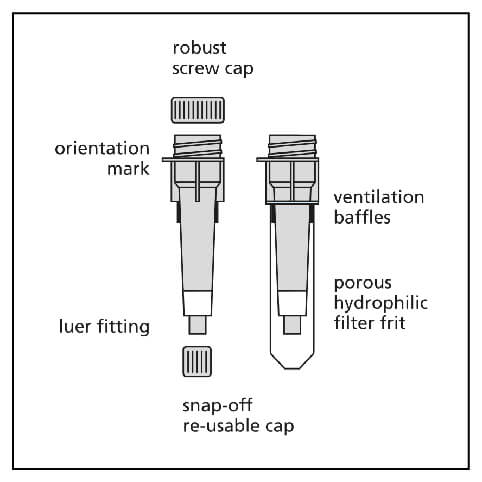
1. Column Preparation
- If the columns have been stored cold, allow to warm to room temperature before use.
- Tap gently or briefly vortex to resuspended gel and remove air bubbles.
- Remove the bottom cap and then remove the top cap.
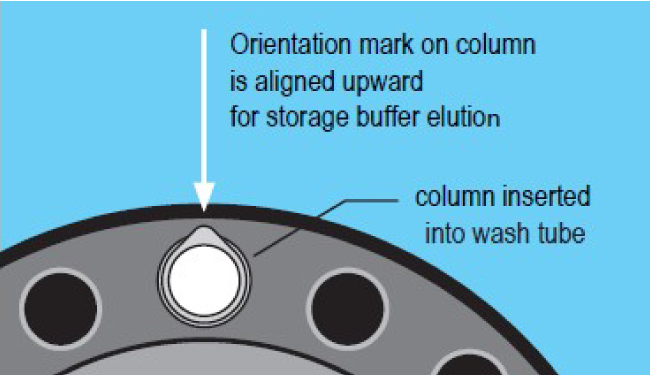
2. Removal of storage buffer
- Place the column into a wash tube.
- Centrifuge at 1000 x g for 2 minutes. Note the column position using the orientation mark.
- Discard wash tube and eluted storage buffer.
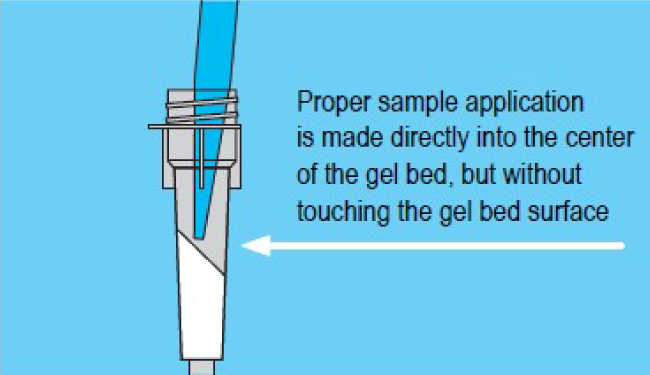
3. Sample Processing
- Carefully apply sample directly to center of gel bed but without touching the gel bed surface.
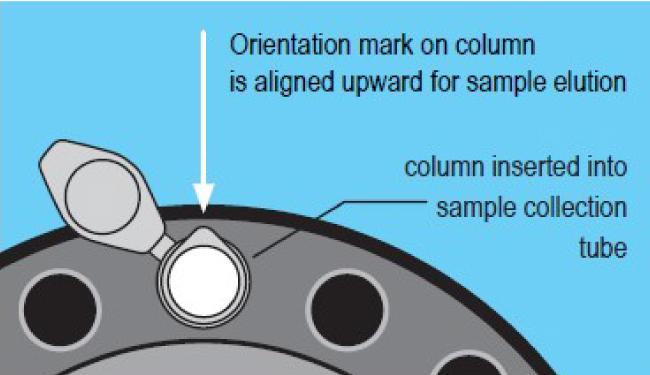
- Place column into a collection tube. Maintain proper column orientation.
- Centrifugate at 1000 x g for 2 minutes to elute the purified sample.
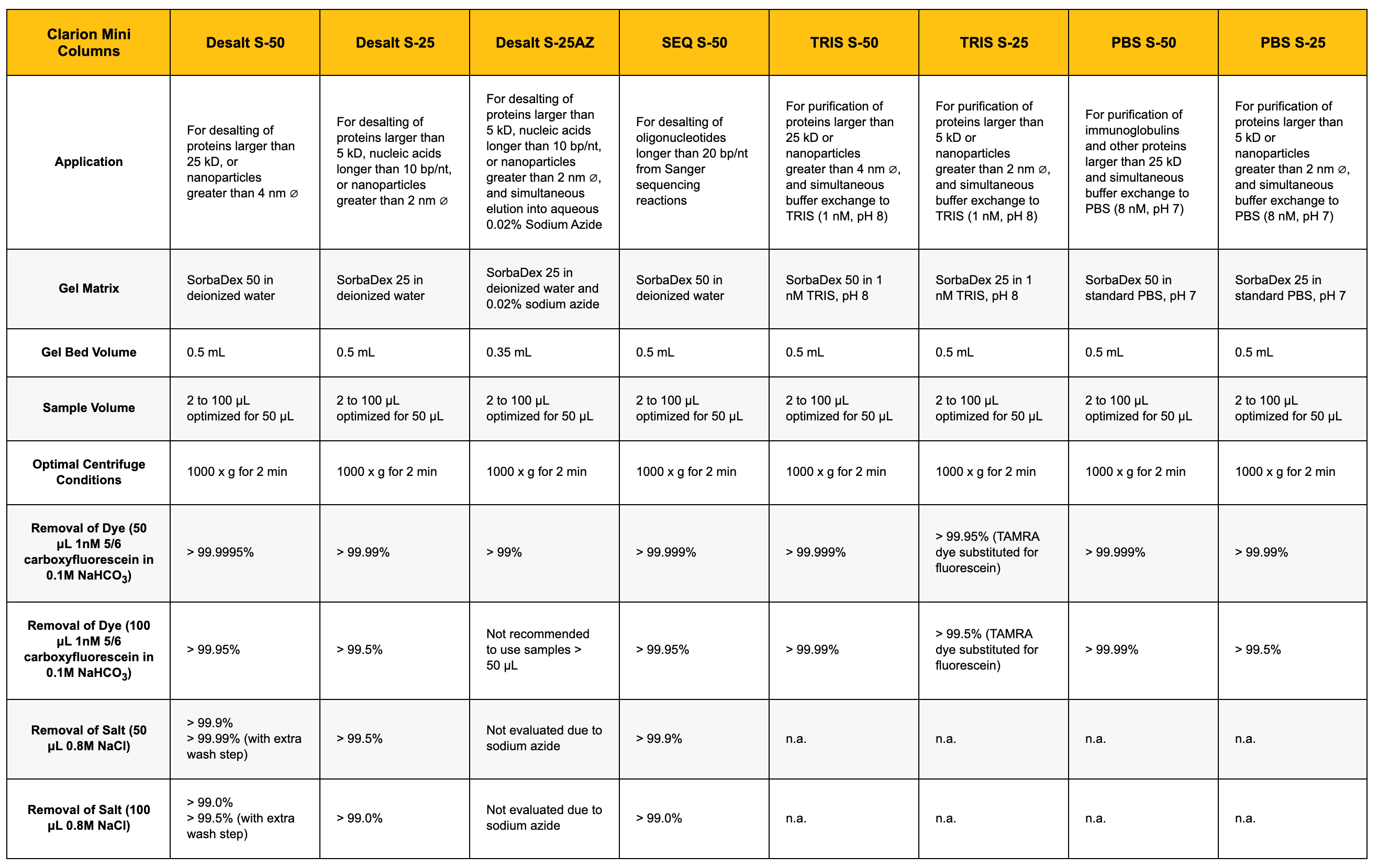
| Clarion Mini Columns | Desalt S-50 | Desalt S-25 | Desalt S-25AZ | SEQ S-50 | TRIS S-50 | TRIS S-25 | PBS S-50 | PBS S-25 |
|---|---|---|---|---|---|---|---|---|
| For desalting of proteins larger than 25 kD, or nanoparticles greater than 4 nm ∅ | For desalting of proteins larger than 5 kD, nucleic acids longer than 10 bp/nt, or nanoparticles greater than 2 nm ∅ | For desalting of proteins larger than 5 kD, nucleic acids longer than 10 bp/nt, or nanoparticles greater than 2 nm ∅, and simultaneous elution into aqueous 0.02% Sodium Azide | For desalting of oligonucleotides longer than 20 bp/nt from Sanger sequencing reactions | For purification of proteins larger than 25 kD or nanoparticles greater than 4 nm ∅, and simultaneous buffer exchange to TRIS (1 nM, pH 8) | For purification of proteins larger than 5 kD or nanoparticles greater than 2 nm ∅, and simultaneous buffer exchange to TRIS (1 nM, pH 8) | For purification of immunoglobulins and other proteins larger than 25 kD and simultaneous buffer exchange to PBS (8 nM, pH 7) | For purification of proteins larger than 5 kD or nanoparticles greater than 2 nm ∅, and simultaneous buffer exchange to PBS (8 nM, pH 7) | |
| SorbaDex 50 in deionized water | SorbaDex 25 in deionized water | SorbaDex 25 in deionized water and 0.02% sodium azide | SorbaDex 50 in deionized water | SorbaDex 50 in 1 nM TRIS, pH 8 | SorbaDex 25 in 1 nM TRIS, pH 8 | SorbaDex 50 in standard PBS, pH 7 | SorbaDex 25 in standard PBS, pH 7 | |
| 0.5 mL | 0.5 mL | 0.35 mL | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL | 0.5 mL | |
| 2 to 100 μL optimized for 50 μL | 2 to 100 μL optimized for 50 μL | 2 to 100 μL optimized for 50 μL | 2 to 100 μL optimized for 50 μL | 2 to 100 μL optimized for 50 μL | 2 to 100 μL optimized for 50 μL | 2 to 100 μL optimized for 50 μL | 2 to 100 μL optimized for 50 μL | |
| 1000 x g for 2 min | 1000 x g for 2 min | 1000 x g for 2 min | 1000 x g for 2 min | 1000 x g for 2 min | 1000 x g for 2 min | 1000 x g for 2 min | 1000 x g for 2 min | |
| > 99.9995% | > 99.99% | > 99% | > 99.999% | > 99.999% | > 99.95% (TAMRA dye substituted for fluorescein) | > 99.999% | > 99.99% | |
| > 99.95% | > 99.5% | Not recommended to use samples > 50 μL | > 99.95% | > 99.99% | > 99.5% (TAMRA dye substituted for fluorescein) | > 99.99% | > 99.5% | |
| > 99.9% > 99.99% (with extra wash step) |
> 99.5% | Not evaluated due to sodium azide | > 99.9% | n.a. | n.a. | n.a. | n.a. | |
| > 99.0% > 99.5% (with extra wash step) |
> 99.0% | Not evaluated due to sodium azide | > 99.0% | n.a. | n.a. | n.a. | n.a. |
Desalt
| Catalog No. | Description | Price (USD) |
|---|---|---|
| 803001-4 | Clarion MINI Spin Columns, Desalt S-25, hydrated with pure, deionized water for quick and efficient desalting, buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 4 columns | |
| 803001-25 | Clarion MINI Spin Columns, Desalt S-25, hydrated with pure, deionized water for quick and efficient desalting, buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 25 columns | |
| 803001-100 | Clarion MINI Spin Columns, Desalt S-25, hydrated with pure, deionized water for quick and efficient desalting, buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 100 columns | |
| 803006-4 | Clarion MINI Spin Columns, Desalt S-50, hydrated with pure, deionized water for quick and efficient desalting, buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 4 columns | |
| 803006-25 | Clarion MINI Spin Columns, Desalt S-50, hydrated with pure, deionized water for quick and efficient desalting, buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 25 columns | |
| 803006-100 | Clarion MINI Spin Columns, Desalt S-50, hydrated with pure, deionized water for quick and efficient desalting, buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 100 columns |
Desalt AZ
| Catalog No. | Description | Price (USD) |
|---|---|---|
| 803005-4 | Clarion MINI Spin Columns, Desalt S-25AZ, hydrated with pure deionized water and stabilized with 0.02% sodium azide for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 4 columns | |
| 803005-25 | Clarion MINI Spin Columns, Desalt S-25AZ, hydrated with pure deionized water and stabilized with 0.02% sodium azide for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 25 columns | |
| 803005-100 | Clarion MINI Spin Columns, Desalt S-25AZ, hydrated with pure deionized water and stabilized with 0.02% sodium azide for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 100 columns |
Tris
| Catalog No. | Description | Price (USD) |
|---|---|---|
| 803003-4 | Clarion MINI Spin Columns, TRIS S-25, hydrated with 1 mM TRIS pH 6 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 4 columns | |
| 803003-25 | Clarion MINI Spin Columns, TRIS S-25, hydrated with 1 mM TRIS pH 6 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 25 columns | |
| 803003-100 | Clarion MINI Spin Columns, TRIS S-25, hydrated with 1 mM TRIS pH 6 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 100 columns | |
| 803008-4 | Clarion MINI Spin Columns, TRIS S-50, hydrated with 1 mM TRIS pH 6 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 4 columns | |
| 803008-25 | Clarion MINI Spin Columns, TRIS S-50, hydrated with 1 mM TRIS pH 6 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 25 columns | |
| 803008-100 | Clarion MINI Spin Columns, TRIS S-50, hydrated with 1 mM TRIS pH 6 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 100 columns |
PBS
| Catalog No. | Description | Price (USD) |
|---|---|---|
| 803004-4 | Clarion MINI Spin Columns, PBS S-25, hydrated with Phosphate Buffered Saline pH 7 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 4 columns | |
| 803004-25 | Clarion MINI Spin Columns, PBS S-25, hydrated with Phosphate Buffered Saline pH 7 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 25 columns | |
| 803004-100 | Clarion MINI Spin Columns, PBS S-25, hydrated with Phosphate Buffered Saline pH 7 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 5 kD, 100 columns | |
| 803009-4 | Clarion MINI Spin Columns, PBS S-50, hydrated with Phosphate Buffered Saline pH 7 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 4 columns | |
| 803009-25 | Clarion MINI Spin Columns, PBS S-50, hydrated with Phosphate Buffered Saline pH 7 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 25 columns | |
| 803009-100 | Clarion MINI Spin Columns, PBS S-50, hydrated with Phosphate Buffered Saline pH 7 for rapid buffer exchange and/or removal of dyes and small molecules from proteins greater than 25 kD, 100 columns |
SEQ S-50
| Catalog No. | Description | Price (USD) |
|---|---|---|
| 803007-4 | Clarion MINI Spin Columns, SEQ S-50, hydrated with pure, deionized water for rapid removal of dyes and dideoxy terminators from sequencing reactions and desalting of oligonucleotides greater than 20 base pairs, 4 columns | |
| 803007-25 | Clarion MINI Spin Columns, SEQ S-50, hydrated with pure, deionized water for rapid removal of dyes and dideoxy terminators from sequencing reactions and desalting of oligonucleotides greater than 20 base pairs, 25 columns | |
| 803007-100 | Clarion MINI Spin Columns, SEQ S-50, hydrated with pure, deionized water for rapid removal of dyes and dideoxy terminators from sequencing reactions and desalting of oligonucleotides greater than 20 base pairs, 100 columns |
| Specifications | ||||||
|---|---|---|---|---|---|---|
| Catalog No. | Product | Solvent | Exclusion Limit (kD) | Exclusion Limit (BP) | Bed Volume (mL) | Optimal Sample Volume (µL) |
Clarion MINI Columns are used for quick and efficient desalting, buffer exchange, and/or removal of dyes and small molecules from proteins (S-25 greater than 5 kD, S-50 greater than 25 kD). Purified proteins are eluted into pure, deionized water (Caution! Some proteins may precipitate in pure water with low ionic strength!) The columns are sterile packed, pre-swollen, and ready-to-use.
Hydrated with pure deionized water and stabilized with 0.02% Sodium azide. Pre-swollen, pre-packed, and ready-to-use.
Clarion MINI Columns are used for quick and efficient desalting, buffer exchange, and/or removal of dyes and small molecules from proteins greater than 5 kD. Purified proteins are eluted into pure, deionized water (Caution! Some proteins may precipitate in pure water with low ionic strength!) The columns are stabilized with 0.02% sodium azide, pre-swollen, and ready-to-use.
Clarion TRIS MINI Columns are used for rapid buffer exchange and/or removal of dyes and small molecules from proteins (S-25 greater than 5 kD, S-50 greater than 25kD). Purified proteins are eluted into 1 mM TRIS, pH 6. The columns are sterile packed, pre-swollen, and ready-to-use.
Hydrated with Phosphate Buffered Saline pH 7. Pre-swollen, pre-packed, and ready-to-use.
Clarion PBS MINI Columns are used for rapid buffer exchange and/or removal of dyes and small molecules from proteins (S-25 greater than 5 kD, S-50 greater than 25 kD). Purified proteins are eluted into Phosphate Buffered Saline (PBS, pH 7). The columns are sterile packed, pre-swollen and ready-to-use.
Hydrated with pure, deionized water. Pre-swollen, pre-packed, and ready-to-use.
Clarion SEQ S-50 MINI Columns are used for rapid removal of dyes and dideoxy terminators from sequencing reactions and for desalting of oligonucleotides greater than 20 base pairs. Purified nucleic acids are eluted into pure, deionized water. The columns are sterile packed, pre-swollen and ready-to-use.


