Selection Criteria and Method Development
By Ray Lombardi, Marc Perla, Rob Cotta, and Robert R. Kerr Ph.D.
Column chromatography method development checklist
1) Phase matching to analyte
- Assess dominant interactions: hydrophobic, polar, ionic, aromatic/π–π, molecular hydrodynamic size range.
- Mode selection:
- Normal phase (NP silica, alumina): Nonpolar analytes; separated by polarity via adsorption. Alumina has a higher polarity than silica for highly polar analytes.
- Reverse phase (RP C18/C8/Phenyl): Polar/ionizable analytes; aqueous organic eluents; improved peak shape for bases/acids.
- Ion exchange (IEX): Strongly ionic analytes; cation/anion exchange with salt or pH elution.
- Size Exclusion (SEC)– For the separation of molecules based on size in an aqueous environment. Best for mixtures in which components differ significantly in molecular weight (e.g., proteins vs. salts, polymers of different chain lengths).
- Stationary phase choices:
- NP: Silica, amino, diol, cyano for tuned polarity and H bonding.
- RP: C18 (general), C8 (faster), phenyl/phenyl-hexyl for π–π selectivity.
- IEX: SCX/SAX cartridges; select capacity for load and target charge.
- SEC: clarify your goal first (desalting, aggregate removal, or true fractionation by size), then Silica for size only in aqueous. Polymer-based for aggregate, desalting, and sizing in a broader pH range in aqueous or organic environments.
- Scaling note: Choose cartridge size based on crude mass and expected load (typically 1–5% of column mass for good resolution).
2) Polarity
- Estimate polarity: logP/logD at working pH; functional groups; H bonding potential.
- Eluent strategy (NP):
- Start strong adsorption: Hexane or heptane.
- Elute with polarity ramps: Gradually increase ethyl acetate, dichloromethane, or acetone.
- Fine tuning: Add small % of alcohol (MeOH/IPA) to break strong adsorption or tailing for polar compounds.
- Eluent strategy (RP):
- Aqueous–organic gradients: Water with ACN or MeOH; add buffer for pH control.
- Selectivity: ACN gives tighter bands and lower backpressure; MeOH alters π–π selectivity.
- Fine tuning: To sharpen peaks add 0.1% formic or 0.1% trifluoroacetic acid
- Practical cue: If the analyte streaks in NP, consider RP or add a polar modifier (1–5% MeOH or 0.1–1% Et3N/AcOH).
3) pKa
- Ionization control: Align eluent pH to favor a single charge state and suppress secondary interactions.
- For acids/bases (RP):
- Favor unionized: Set pH ≥ pKa + 2 (acids) or ≤ pKa − 2 (bases) for cleaner bands and stronger retention.
- Buffers: Volatile (ammonium acetate/formate) for MS friendly fractions; phosphate for UV/bench work.
- For NP silica:
- Basic analytes: Add 0.1–1% triethylamine to deactivate silanols and reduce tailing.
- Acidic analytes: Add 0.1–1% acetic acid to moderate hydrogen bonding and improve symmetry.
- Avoid pH ≈ pKa: Mixed species can cause split bands, shoulders, and variable Rf/Rt.
4) Solubility
- Load solution compatibility: Use a solvent that dissolves the crude at practical concentration (10–200 mg/mL) and is equal or weaker in elution strength than the initial mobile phase.
- NP best practices:
- Preferred loaders: DCM, ethyl acetate, toluene, minimal MeOH; avoid neat DMF/DMSO.
- Dry load option: Adsorb crude onto silica or Celite for very polar or salty mixtures; pack as a plug.
- RP best practices:
- Preferred loaders: Water/ACN or water/MeOH mixes; match initial gradient composition.
- pH assistance: Dissolve in ionized form, then adjust to working pH before loading.
- Additives: Keep nonvolatile salts low; DMSO ≤2–5% only if necessary; filter through 0.45–0.2 µm to prevent channeling.
5) Molecular weight
- Small molecules (≤1,000–1,200 Da): Standard 60 angstrom pore silica or C18 cartridges (20–120 g) with typical flow rates; expect sharp bands. With RP cartridges, use 100 A to counter any steric hindrance and improve interaction with the bonded phase.
- Larger molecules/Biomolecules: Use wider pore sorbents 100-300A, lower flow rates, and gentler gradients; consider IEX for charge driven separation.
- Loading and resolution:
- Rule of thumb: 1–5% of cartridge mass as analyte; reduce load for closely eluting impurities.
- Fraction timing: Shorter collection windows for low MW, broaden for higher MW (slower mass transfer).
6) Mobile phase compatibility
- Detector compatibility: UV at 210–254 nm common; avoid eluents that absorb strongly at target λ.
- Volatility and post processing: Prefer volatile systems if fractions go to MS or require easy evaporation (hexane/EtOAc, ACN/water).
- Hardware/pH limits: Respect cartridge manufacturer pH ranges (RP often 2–10; silica NP not used with strong aqueous buffers).
- Safety and handling: Manage halogenated solvents, static buildup, and waste segregation; degas RP solvents to reduce baseline noise if using UV.
7) Scouting and optimization workflow
- Thin layer chromatography (TLC) first:
- TLC solvent screens: Hexane/EtOAc ladder (0–100%), DCM/MeOH (0–10%), ACN/MeOH/water (RP mimic).
- Target Rf: 0.2–0.35 for the analyte; adjust solvent strength accordingly.
- Column/cartridge selection:
- NP: 40-75 µm silica or higher resolution 20-45 µm ; Granular for general separations and lower costs. Spherical for higher resolution, greater efficiencies, improved reproducibility, and decreased backpressure. Base size on crude mass.
- RP: C18 cartridges; select pore and particle size suitable for MW and viscosity. Pore size great than 90A. 40-75 µm silica or higher resolution 20-45 µm
- Gradient design:
- NP example: Hexane/EtOAc 0–40% over 20 column volumes; optional 0.5% Et3N.
- RP example: 5–40% ACN in water (0.1% formic or 10 mM ammonium acetate) over 15–20 CV.
- Flow and pressure: Start moderate (per cartridge spec), increase gradually while watching band integrity; avoid channeling.
- Monitoring: UV channels (e.g., 210/254 nm), ELSD if needed; confirm fractions by TLC or rapid LC/MS.
- Optimization levers:
- Polarity: Adjust solvent strength in small steps (5–10% increments NP; 3–5% organic RP).
- pKa/pH: Add or titrate modifiers (Et3N/AcOH NP; buffer and pH RP).
- Selectivity: Swap ACN↔MeOH in RP or EtOAc↔DCM in NP to shift aromatic/π and H bond interactions.
8) Robustness, documentation, and acceptance criteria
- System suitability (flash context):
- Before run: Stable baseline, consistent backpressure, clean blank elution, reproducible TLC Rf.
- Robustness checks:
- Solvent strength: ±5–10% changes maintain resolution.
- Load amount: ±20–30% without band broadening or breakthrough.
- Modifier level: ±0.2–0.5% (Et3N/AcOH) maintains peak symmetry.
- Acceptance criteria:
- Purity in pooled fractions: ≥95% by TLC/LC/MS.
- Recovery: ≥80% unless highly polar/basic (document exceptions).
- Band integrity: No tailing beyond 1–2 CV; minimal overlap with nearest impurity.
- Documentation:
- Record per concern: Phase/mode choice, polarity evidence (TLC/solvent ladder), pKa rationale and modifiers, solubility and loader, MW driven cartridge size, and mobile phase compatibility.
- Final method sheet: Cartridge type/size, gradient program, solvents/additives and percentages, flow rate, detection wavelengths, collection window, work up instructions.
Quick Reference Matrix:
| Concern | What to assess | Primary decision | Typical actions |
|---|---|---|---|
| Phase matching to analyte | Dominant interactions | NP vs RP vs IEX vs SEC | Choose cartridge chemistry and mode |
| Polarity | Rf, logD, H-bonding | Eluent strength & type | Adjust hexane/EtOAc or RP ACN/MeOH |
| pKa | Ionization vs pH | Modifiers and buffers | Et3N/AcOH (NP); buffer pH (RP) |
| Solubility | Loader vs gradient start | Focusing & band shape | Dry load; match weak loader |
| Particle Shape | Granular vs Spherical | Cost vs Load vs. Resolution | Granular for Cost, Spherical for resolution |
| Molecular weight | Size & diffusion | Cartridge scale & pore | Set load %, select pore size |
| Mobile phase compatibility | Detector/evaporation | Solvent & additive choice | Volatile systems; UV windows |
Sample Loading and Flow Rate Considerations
Determining Sample Load
The maximum sample load in column or flash chromatography depends on the adsorbent bed capacity, column dimensions, and the complexity of the solute mixture.
- Column bed volume and particle size: Larger columns with higher surface area can accommodate greater sample loads without significant loss of resolution. Finer particle adsorbents increase loading capacity but also raise backpressure.
- General Loading Guidance: For silica gel flash chromatography, typical loading is 1–5% of the column’s silica mass for crude mixtures, and up to 10% for relatively pure compounds.
- Pilot runs: A small-scale test injection helps determine whether peak broadening or co-elution occurs at a given load. Use a series of increasing injection loads. When resolution deteriorates (maximum load vol.), the load must be reduced, or the column size increased.
- Sample solubility: The sample must be dissolved in a minimal volume of a solvent compatible with the mobile phase. Overly concentrated or insoluble samples can cause band distortion. Too high a solvent volume lowers analyte concentrations, potentially compromising resolution.
Flow Velocity Considerations
Column dimensions, particle size, and system pressure limits govern flow velocity.
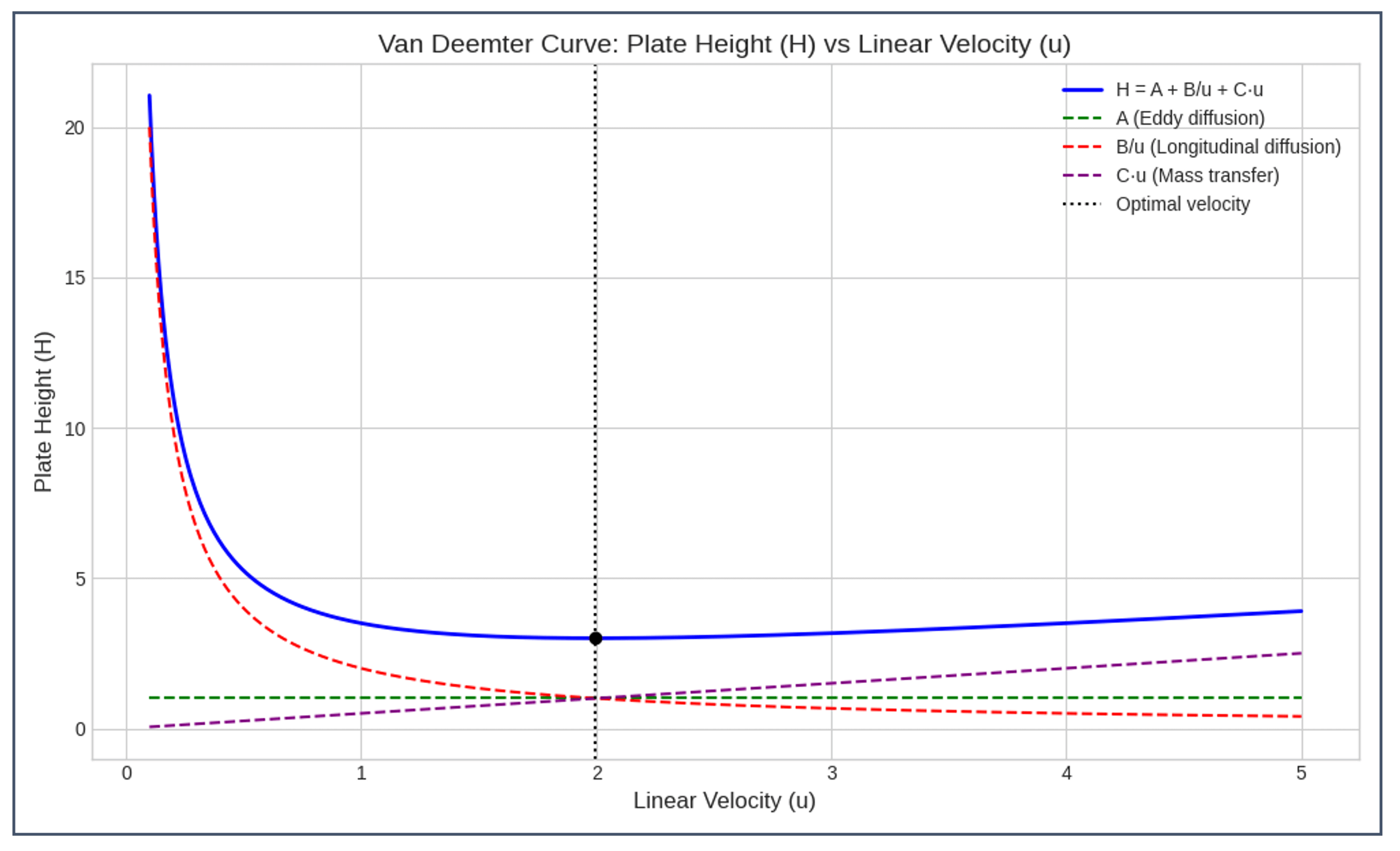
- Linear velocity (cm/min): More significant than volumetric flow rate, as it normalizes for column diameter.
- Van Deemter equation: Resolution is optimized at intermediate velocities. Too slow a flow increases diffusion broadening, while too fast a flow reduces interaction time, resulting in broadened peaks.
- Flash chromatography guidance: Flow rates should be set to produce 2–5 column volumes per hour. Higher flow velocities will shorten run times but will increase the risk of separation inefficiency.
- Pressure limits: Smaller particle sizes (<20 µm) require lower velocities to avoid excessive backpressure, while larger particles tolerate faster flows.
Tuning Resolution vs. Throughput- Resolution vs. Cost
- Resolution priority: Lower sample load, slower flow velocity, and longer column length improve separation. This is critical for complex mixtures or when purity is paramount.
- Throughput priority: Higher flow rates and larger sample loads maximize productivity, but resolution may suffer. This is acceptable for routine separations where minor impurities are tolerable.
- Gradient optimization: Steeper solvent gradients increase throughput but reduce resolution; shallow gradients enhance resolution but extend run time.
Balancing Time vs. Cost
- Time efficiency: Faster runs reduce labor and instrument occupancy but may require larger columns or more solvent to compensate for reduced resolution.
- Cost efficiency: Solvent consumption is a significant cost driver. Slower runs with shallow gradients consume more solvent, while faster runs with steeper gradients use less solvent but may affect purity recovery levels.
- Scaling decisions: For preparative work, cost per gram of purified product often dictates the balance. High-value compounds justify slower, more solvent-intensive runs; bulk/easy separations favor speed and lower solvent use.
Suggested Starting Strategy
- Start with small-scale scouting runs to determine load tolerance and gradient steepness.
- Adjust flow velocity to balance resolution and run time, guided by the Van Deemter curve.
- Scale column dimensions proportionally when increasing load to maintain separation quality.
- Evaluate solvent consumption against product value to decide whether resolution or cost efficiency is the priority.
General Loading Reference for Silica Flash Cartridges
Note: Alumina cartridges typically allow lower load percentages (0.1–4%) than silica cartridges.
| Cartridge Size (Silica Mass) | Column Volume (mL) | Typical Max Load (mg–g) | Practical Range (% of sorbent mass) |
|---|---|---|---|
| 4 g | ~6 mL | 4 mg – 0.4 g | 0.1–10% |
| 12 g | ~20 mL | mg – 1.2 g | 0.1–10% |
| 25 g | ~30 mL | 25 mg – 2.5 g | 0.1–10% |
| 40 g | ~50 mL | 40 mg – 4.0 g | 0.1–10% |
| 80 g | ~110 mL | 80 mg – 8.0 g | 0.1–10% |
| 120 g | ~155 mL | 120 mg – 12 g | 0.1–10% |
| 220 g | ~280 mL | 220 mg – 22 g | 0.1–10% |
| 330 g | ~430 mL | 330 mg – 33 g | 0.1–10% |
| 800 g | ~1050 mL | 0.8 g – 80 g | 0.1–10% |
| 1600 g | ~2000 mL | 1.6 g – 160 g | 0.1–10% |
| 3000 g | ~3910 mL | 3.0 g – 300 g | 0.1–10% |
| 5000 g | ~5800 mL | 5.0 g – 500 g | 0.1–10% |
| Scenario | Sample Load | Flow Rate | Gradient Steepness | Solvent Use |
|---|---|---|---|---|
| Resolution-focused | Low | Slow | Shallow | High |
| Throughput-focused | High | Fast | Steep | Moderate |
| Cost-focused | Moderate | Moderate | Balanced | Low |


