Better, Faster Separations for Closely Eluting Peaks
When compounds elute too closely together, purification becomes inefficient. That’s where gradient elution comes in. This technique gradually changes the mobile phase composition—from weak to strong—over the course of a separation.
The most common approach is a linear gradient, where the solvent composition shifts at a constant rate. In many cases, a simple linear gradient provides enough resolution to separate your compounds effectively.
But What If a Linear Gradient Isn’t Enough?
That’s when it’s time to use a step or focused gradient.
A step gradient uses a series of isocratic holds with defined mobile-phase combinations. These “steps” pause the solvent change at key intervals, giving certain compounds the extra resolution they need. The result is sharper separations without lengthening the overall run time—and often with reduced solvent consumption.
In short, step gradients offer a smarter, more efficient path when linear gradients alone don’t deliver.
Examples of Gradients
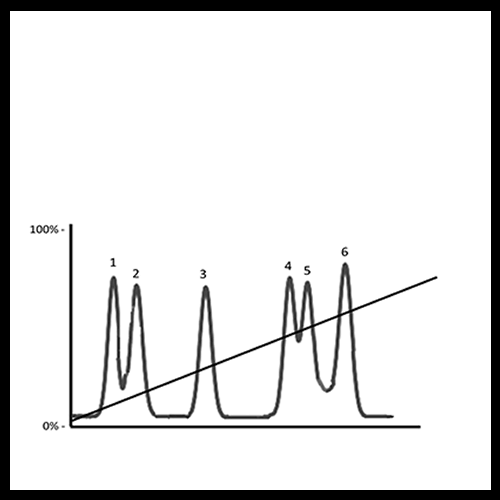
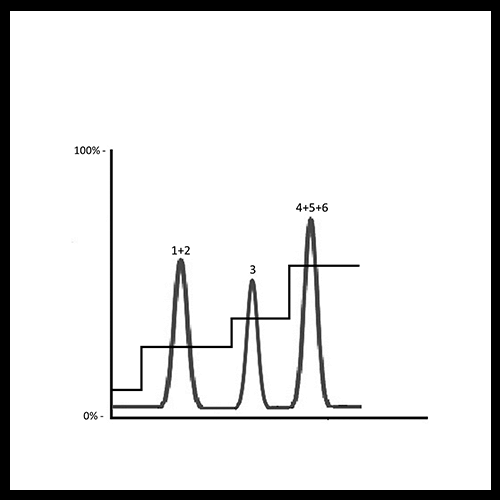
In challenging separations, a step gradient offers several advantages. It can shorten run times, reduce the distance between eluting species, and still improve the resolution of closely eluting peaks. Moreover, it helps isolate the peak of interest from other components in the mixture, making the overall process more efficient. Beyond the lab, these benefits become even more significant at the industrial scale, where time savings and improved resolution directly translate into higher productivity and lower costs.
Isolation of a Peak of Interest
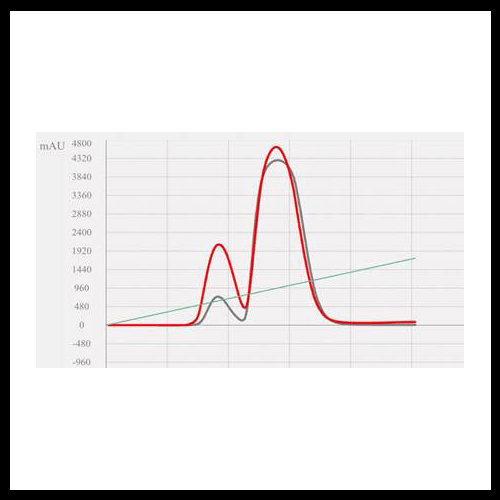
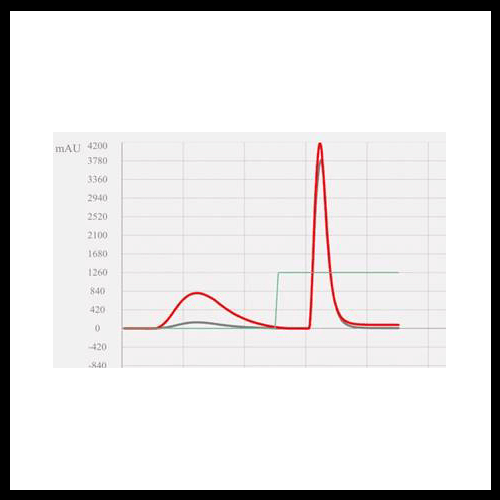
Why Timing Matters
It may seem natural to add an isocratic hold based on the elution time of your peak of interest. However, this does not work. By the time you see the peak in the chromatogram, it has already passed through the column and reached the detector. Any change made at this point happens after separation, not during.
Know Your Volumes
To set a proper step gradient, you need to know both the system volume and the column volume. These values reveal the offset between when the gradient begins and when the detector registers the peaks. With this information and your flow rate, you can calculate the actual solvent strength that caused separation—not just detection.
How to Set the Gradient
Start your step about 5% below the solvent strength that eluted the peak in your scouting run. Then, end the step about 5% above that value. This focused range improves resolution and keeps separations tighter.
Calculating Hold Time
Always calculate hold time in column volumes (CV). Base it on the apex of your target peak. Add extra CVs so the step begins just before the peak and finishes after it. This ensures you capture the full separation window.
Use Automation to Simplify
Modern flash systems make this much easier. Many instruments can now create step or focus gradients automatically using data from scouting runs or TLC.
The SepaBean Automated Flash System goes even further. It suggests columns, recommends gradients, and programs steps based on user-supplied TLC or HPLC data.
Beyond Gradient Control
SepaBean also includes smart features such as method recommendations, fraction tracking, and compliance tools. Together, these make purification faster, more reliable, and easier for both routine and complex workflows.



